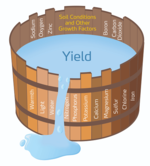
Liebig's barrel is put in like every presentation on plant growth:

en.wikipedia.org
When adding fertilizer, you just want to make sure one of your nutrients isn't your bottleneck. That's why often adding 10-10-10 doesn't make sense.
Plants likely remove fertilizer in a 3:1:2 ratio. Additionally, phosphorous is least likely to completely wash out from your substrate. Though I haven't seen any specific bonsai data on that. Could be interesting.
I know very little about plant ecology, but my understanding is that plant species can change extremely abruptly when the geology changes. Like with almost no transition. Because of how plants are able to take up minerals as they are specialized for one type of geology, and not the other. Even when in culture the difference in species and their preference isn't that big, natural selection will amplify this change.
The botany guy with the strong Chicago accent on Youtube often explains this. The 'crime pays, botany doesn't' guy.
Need to remember that we measure fertiliser ratios differently in Australia and, I think, in NZ and some other countries. USA measures the percentage of the compound eg K in fertilisers usually comes from K2O and P is often P2O5. The O in those compounds weighs something but is not used by the plants. Down here the fertiliser ratios measure only the elemental part of the P and K so a fertiliser with the same ratio numbers here actually has more P and K than one with that ratio sold in the USA.
There's not a huge difference but maybe worth knowing?
Not quite. NPK ratios are always expressed as if P comes from P2O5 and K comes from K2O. Even thought P2O5 and K2O don't exist as actual chemicals. I believe this is basically universal across the entire world and this way of expressing is enforced by regulatory bodies. Of course elemental phosphorus exists. And the phosphate ion exists. But not P2O5, whatever that would be. Phosphorus pentoxide does exist, though. But that is P4O10. P2O5 is used as a proxy. It doesn't exist. Usually, a fertilizer would contain mono potassium phosphate. And then a bunch of animal waste products high in nitrogen, like blood meal, feather meal. And then ammonium nitrate or potassium nitrate are added to get the desired NPK ratio.
Any resource on fertilizer will have a table for converting say P2O5 to actual phosphate ion concentrations. If you are like a normal person, you want to know the molar concentration of phosphate PO4 3-.